GlaxoSmithKline Plc is set to start working on an experimental vaccine against the Ebola virus, Reuters has reported.
In a separate development, China on Friday sentenced a UK investigator for having illegally obtained private records of Chinese citizens and selling the information on to clients including the FTSE 100 drugmaker.
GSK’s share price has added just under 0.5 percent in early morning trading in London.
Ebola vaccine trials
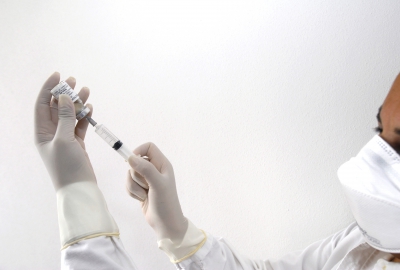
Reuters reported yesterday that GSK was set to launch a clinical trial of an experimental vaccine against the Ebola virus which has killed nearly 1,000 people in West Africa.
A GSK spokeswoman told the newswire that the trial should get underway “later this year”. The vaccine has produced promising results in animal studies involving primates and is due to enter initial Phase I testing in humans, pending approval from the US Food and Drug Administration (FDA).
“It is right at the beginning of the development journey and still has a very long way to go,” the GSK official told Reuters, declining to comment on a possible timeline for launch.
The drugmaker’s partner, the US National Institute of Allergy and Infectious Disease (NIAID), said in a statement that the early-stage trial could start “as early as fall 2014”.
The NIAID explained that the vaccine contained no infectious Ebola virus material, but was instead based on a chimpanzee adenovirus, into which two Ebola genes had been inserted.
GSK acquired the vaccine after buying Swiss biotech company Okairos for €250 million (£199 million) last year.